Tumor Cells
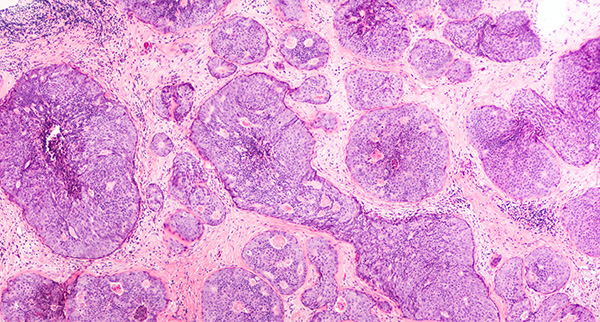
Tumor cells proliferate uncontrollably to form malignant, premalignant, or benign tissue masses. Malignant cells evade the signals that balance cell division by apoptosis, allowing characteristically fast and unregulated tumor growth.1 They are able to travel around the body as either single migratory circulating tumor cells (CTCs) or clusters of CTCs, acting as precursors for metastasis and secondary tumor development.2
Formation and Structure
Tumor cells arise from the accumulation of mutations in the various gene networks that control cell proliferation. Alterations to the expression of cancer-associated genes, such as the dysregulation of tumor suppressor genes or the activation of oncogenes, can lead to uncontrolled cell division.3 Epigenetic histone modifications and disruption of chromatin remodelling machinery have also been shown to play important roles in tumor cell formation.4 These alterations ultimately lead to tumorigenesis, which is the gradual loss of normal cell function and increase in malignant properties including metastasis, angiogenesis, and evasion of apoptosis.
There are many morphological and functional differences between healthy tissue cells and cancerous tumor cells that help clinicians to determine the malignant potential of tumors. Cancer cells are characterized by irregular size and shape, with greater cell-to-cell variability, an abnormally large nucleus, prominent nucleoli, and a scarce cytoplasm.5
Function
Evading Apoptosis
Cancerous tumor cells can survive by modulating apoptotic pathways to avoid cell death. Tumor cells evade apoptosis by increasing or decreasing the expression of anti- or pro-apoptotic genes, respectively. They can also avoid apoptosis by altering the stability of anti- or pro-apoptotic proteins or adjusting their functions through post-translational modifications.6
Eluding Immunity
Tumor cell survival also relies heavily on evading the immune system by avoiding recognition or maintaining an immunosuppressive tumor microenvironment (TME).7 Malignant cells may lose the ability to express cell surface antigens and/or downregulate antigen-presenting machinery, avoiding recognition by cytotoxic T cells and conferring resistance to effector molecules.8 Tumor cells can also create an immunotolerant TME by secreting immunosuppressive molecules, expressing inhibitory checkpoint molecules, or inducing the recruitment of T regulatory (Treg) cells with chemokines.7 These Treg cells are responsible for suppressing the activation of cytotoxic effector immune cells, such as T helper cells (Th1) and natural killer cells, to avoid an immune response.9 Immune checkpoint ligands, including PDL1/L2, CD47, CD155(PVR), CD112 and CEACAM-1, bind to T cell receptors to suppress anti-tumor response and allow tumor cells to escape immune surveillance.10
Angiogenesis
Tumor cells develop neovessels from preexisting blood vessels via angiogenesis to deliver essential nutrients and oxygen for growth, and provide cells with an exit route into the bloodstream. Vascular homeostasis is typically maintained by a balance of pro- and anti-angiogenic factors. Upregulation of pro-angiogenic signaling in tumors sparks blood vessel formation and activates endothelial cells, releasing malignant cells from dormancy and promoting tumor growth.11
Metastasis
Metastasis is a hallmark of malignancies that develops as cells spread from the primary tumor to other tissues and organs, accounting for 90 % of patient mortality in solid cancers.12 The process to metastasis begins with invasion of extracellular matrices into surrounding tissues, coupled by angiogenesis. Intravasation into the vascular system allows CTCs to be transported around the body, until they reach other tissues, where they may proliferate to establish a secondary tumor or remain dormant.13,14 Research has also shown that CTCs can travel in clusters with neutrophils, which helps cell cycle progression and leads to more aggressive metastasis formation.15
Emerging Research
Research is increasingly finding new and more reliable ways to diagnose, destroy and prevent tumor cells, and ultimately improve the therapeutic outcome of cancer treatments. Huge advances have been made in the field of personalized medicine, including CAR-T16, CAR-NK17 and CAR-M18 therapies, both of which reprogram the body’s own immune system to attack tumor cells.19
Tools to Study Tumor Cells
The combination of surface cell marker and cell cycle analyses using flow cytometry can discriminate normal cell populations from tumor cells and is useful for investigating the underlying mechanism of tumor growth and evaluating the researching potential treatments.20,21
CTCs are potent indicators of metastasis that can be captured and enriched from liquid biopsies, before being distinguishing from other cell types using immunofluorescence or various PCR-based techniques.22 Cell sorting technology can aide the study of various types of CTCs by flexible addition of fluorescent labels and isolation as single cells for further molecular analysis.23 Novel microfluidic devices have also been developed to enrich and detect CTCs, while minimizing sample consumption.24 High-resolution temporal and spatial imaging can be used to track the real-time dynamic movements of CTCs through metastasis and interpret interactions between cells.25 Scientists are also developing 3D in vitro models of tumoroids, spheroids, and organoids to study entire TMEs and the interaction between tumor and immune cells.26
Cell Markers
The cell markers used for detecting tumor cells can vary between each cell line or cancer type, and often only identify tumor cells if associated with aberrant patterns of other co-expressed makershe most common cell markers for CTCs are pan-epithelial cell markers EpCAM and cytokeratins.27,28 In addition, the metastatic properties of tumor cells can be investigated with vimentin as a marker for epithelial-mesenchymal transition (EMT), with its overexpression correlating with tumor growth and metastasis.29
Related Products
References
1. Fulda S, Debatin KM. Apoptosis signaling in tumor therapy. (2004) Annals of the New York Academy of Science. 1028:150-6. doi: 10.1196/annals.1322.016. PMID: 15650241.
2. Rushton AJ, Nteliopoulos G, Shaw JA, Coombes RC. A Review of Circulating Tumour Cell Enrichment Technologies. Cancers (Basel). 2021 Feb 26;13(5):970. doi: 10.3390/cancers13050970. PMID: 33652649; PMCID: PMC7956528.
3. Curtius, K., Wright, N. A., & Graham, T. A. (2017). Evolution of Premalignant Disease. Cold Spring Harbor perspectives in medicine, 7(12), a026542. https://doi.org/10.1101/cshperspect.a026542
4. Muntean, A. G., & Hess, J. L. (2009). Epigenetic dysregulation in cancer. The American journal of pathology, 175(4), 1353–1361. https://doi.org/10.2353/ajpath.2009.081142
5. Baba AI, Câtoi C. Comparative Oncology. Bucharest (RO): The Publishing House of the Romanian Academy; 2007. Chapter 3, TUMOR CELL MORPHOLOGY. Available from: https://www.ncbi.nlm.nih.gov/books/NBK9553/
6. Sharma, A., Boise, L. H., & Shanmugam, M. (2019). Cancer Metabolism and the Evasion of Apoptotic Cell Death. Cancers, 11(8), 1144. https://doi.org/10.3390/cancers11081144
7. Gonzalez, H., Hagerling, C., & Werb, Z. (2018). Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes & development, 32(19-20), 1267–1284. https://doi.org/10.1101/gad.314617.118
7. Gonzalez, H., Hagerling, C., & Werb, Z. (2018). Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes & development, 32(19-20), 1267–1284. https://doi.org/10.1101/gad.314617.118
7. Gonzalez, H., Hagerling, C., & Werb, Z. (2018). Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes & development, 32(19-20), 1267–1284. https://doi.org/10.1101/gad.314617.118
8. de Charette M, Marabelle A, Houot R. (2016) Turning tumour cells into antigen presenting cells: The next step to improve cancer immunotherapy? European Journal of Cancer. 68:134-147. DOI: 10.1016/j.ejca.2016.09.010. PMID: 27755997.
9. Ward‐Hartstonge, K. A., & Kemp, R. A. (2017). Regulatory T‐cell heterogeneity and the cancer immune response. Clinical & translational immunology, 6(9), e154.
10. Hu, M., Li, Y., Lu, Y., Wang, M., Li, Y., Wang, C., Li, Q., & Zhao, H. (2021). The regulation of immune checkpoints by the hypoxic tumor microenvironment. PeerJ, 9, e11306. https://doi.org/10.7717/peerj.11306
11. Lugano, R., Ramachandran, M. & Dimberg, A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 77, 1745–1770 (2020). https://doi.org/10.1007/s00018-019-03351-7
12. Jiang WG, Sanders AJ, et al. Tissue invasion and metastasis: Molecular, biological and clinical perspectives. Semin Cancer Biol. 2015 Dec;35 Suppl:S244-S275. doi: 10.1016/j.semcancer.2015.03.008. Epub 2015 Apr 10. PMID: 25865774.
13. Micalizzi, D. S., Maheswaran, S., & Haber, D. A. (2017). A conduit to metastasis: circulating tumor cell biology. Genes & development, 31(18), 1827–1840. https://doi.org/10.1101/gad.305805.117
14. Bielenberg, D. R., & Zetter, B. R. (2015). The Contribution of Angiogenesis to the Process of Metastasis. Cancer journal (Sudbury, Mass.), 21(4), 267–273. https://doi.org/10.1097/PPO.0000000000000138
15. Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, Scheidmann MC, Donato C, Scherrer R, Singer J, Beisel C, Kurzeder C, Heinzelmann-Schwarz V, Rochlitz C, Weber WP, Beerenwinkel N, Aceto N. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019 Feb;566(7745):553-557. doi: 10.1038/s41586-019-0915-y. Epub 2019 Feb 6. PMID: 30728496.
16. Mehrabadi, A. Z., Ranjbar, R., Farzanehpour, M., Shahriary, A., Dorostkar, R., Hamidinejad, M. A., & Ghaleh, H. E. G. (2022). Therapeutic potential of CAR T cell in malignancies: A scoping review. Biomedicine & Pharmacotherapy, 146, 112512.
17. Wang, W., Jiang, J., & Wu, C. (2020). CAR-NK for tumor immunotherapy: Clinical transformation and future prospects. Cancer letters, 472, 175-180.
18. Moradinasab S, Pourbagheri-Sigaroodi A, Ghaffari SH, Bashash D. Targeting macrophage-mediated tumor cell phagocytosis: An overview of phagocytosis checkpoints blockade, nanomedicine intervention, and engineered CAR-macrophage therapy. International Immunopharmacology. 2021 Dec;103:108499. DOI: 10.1016/j.intimp.2021.108499. PMID: 34972068.
19. Ahn, J. C., Teng, P. C., Chen, P. J., Posadas, E., Tseng, H. R., Lu, S. C., & Yang, J. D. (2021). Detection of Circulating Tumor Cells and Their Implications as a Biomarker for Diagnosis, Prognostication, and Therapeutic Monitoring in Hepatocellular Carcinoma. Hepatology, 73(1), 422-436.
20. Kim, K. H., & Sederstrom, J. M. (2015). Assaying Cell Cycle Status Using Flow Cytometry. Current protocols in molecular biology, 111, 28.6.1–28.6.11. https://doi.org/10.1002/0471142727.mb2806s111
21. Cobb, L., & Das, S. (2013). The Cell cycle analysis. MATER METHODS. 3:172. Accessed: https://www.labome.com/method/The-Cell-Cycle-Analysis.html
22. Lopresti, A., Malergue, F., Bertucci, F., Liberatoscioli, M. L., Garnier, S., DaCosta, Q., Finetti, P., Gilabert, M., Raoul, J. L., Birnbaum, D., Acquaviva, C., & Mamessier, E. (2019). Sensitive and easy screening for circulating tumor cells by flow cytometry. JCI insight, 5(14), e128180.
23. Lopresti A, Malergue F, Bertucci F, Liberatoscioli ML, Garnier S, DaCosta Q, Finetti P, Gilabert M, Raoul JL, Birnbaum D, Acquaviva C, Mamessier E. Sensitive and easy screening for circulating tumor cells by flow cytometry. JCI Insight. 2019 Jun 13;5(14):e128180. doi: 10.1172/jci.insight.128180. PMID: 31194699; PMCID: PMC6675556.
24. Hu, X., Zang, X., & Lv, Y. (2021). Detection of circulating tumor cells: Advances and critical concerns. Oncology letters, 21(5), 422. https://doi.org/10.3892/ol.2021.12683
25. Su, S., & Li, X. (2021). Dive into Single, Seek Out Multiple: Probing Cancer Metastases via Single-Cell Sequencing and Imaging Techniques. Cancers, 13(5), 1067. https://doi.org/10.3390/cancers13051067
26. Shelton, S. E., Nguyen, H. T., Barbie, D. A., & Kamm, R. D. (2020). Engineering approaches for studying immune-tumor cell interactions and immunotherapy. iScience, 24(1), 101985. https://doi.org/10.1016/j.isci.2020.101985
27. Welinder C, Jansson B, Lindell G, Wenner J. Cytokeratin 20 improves the detection of circulating tumor cells in patients with colorectal cancer. Cancer Lett. 2015 Mar 1;358(1):43-6. doi: 10.1016/j.canlet.2014.12.024. Epub 2014 Dec 17. PMID: 25528628.
28. Ulrich H, Tárnok A. Flow cytometry detection of circulating tumor cells: achievements and limitations as prognostic parameters. Cytometry A. 2014 Mar;85(3):201-2. doi: 10.1002/cyto.a.22441. PMID: 24535954.
29. Mittal V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu Rev Pathol. 2018 Jan 24;13:395-412. doi: 10.1146/annurev-pathol-020117-043854. PMID: 29414248.

